Can we measure cognition in a translatable way across species?
Significant advances in genetics have enabled the creation of numerous mouse lines modeling genetic variants associated with a variety of neuropsychiatric diagnoses. However, it has been incredibly difficult to understand how or why a given genetic variant might promote a particular pattern of complex cognition. In part, that is because the suite of behavioral tasks usually employed to study mice measure very simple behaviors that aren’t always adequate to access complex cognitive domains such as executive function.
Our lab has a particular interest in autism spectrum disorders (ASD), which exemplifies the situation. There has been an explosion in the generation of mice modeling autism-associated genotypes, but without a matched increase in the sophistication of behavioral testing.
Autism is a complex diagnosis, involving social difficulties, challenges in tackling unpredictable environments, and repetitive behaviors. In part, these traits may be driven by difficulties in predicting what is meant in an ambigious location or from an ambiguous social interaction. The ability to predict and plan based on likely states of the world is a key aspect of executive function, and people on the spectrum report executive function issues as a particular problem from their perspective. How can we move past simple behaviors to access these complex cognitive domains in mice?
Using a number of cognitive tasks probing executive function, we tease out the relationships between outcome prediction, motivation, and goal-directed behavior in mouse models of neuropsychiatric vulnerability. These include well-established tasks such as several varieties of bandit task, delay discounting, probability discounting/risky decision making, and the 5-choice serial reaction time task and other continuous performance tasks. We are also interested in back-translation of novel human tasks into rodent models.
Autism: Our lab has found that mice who were developed to express genetic variants associated with autism have difficulties with predicting the positive outcomes of their actions, have trouble predicting changes in the association between an action and a reward, and have profound differences in catecholamine function. These effects are often strongest in male mice carrying autism-associated genotypes. We are actively pursuing projects to measure neural activity and neurotransmitter release in corticostriatal regions in mouse models of autism associated genotypes during these tasks. Further, by manipulating the function of specific neurons via optogenetic and chemogenetic approaches, we wish to define the circuits that are disrupted (and those that are resilient) in these mice.
Psychosis: We are actively pursuing the testing of animal models in novel, psychosis-relevant cognitive tasks.
Early life environment: Dr. Grissom has extensive experience testing early life environmental challenges on cognition in adult offspring, and is interested in pursuing models associated with broad neurodevelopmental outcomes.
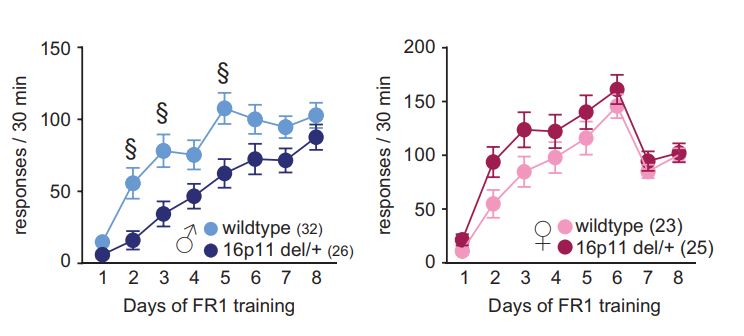
A copy number on chromosome 16 (resulting in the loss of one copy of the 16p11.2 region) profoundly increases the risk of diagnosis of neurodevelopmental disorders, particularly autism and ADHD, in humans. Human 16p11.2 deficiency syndrome can be very closely modeled in mice via hemideletion of chromosome 7qF3 , as the genetic architecture of this region is highly conserved.
In our previous research, we found that a mouse model of 16p11.2 hemideletion shows male-specific impairments in operant learning under a fixed-ratio schedule.